What is Brown’s Gas HHO?
Brown’s Gas HHO, also known as oxyhydrogen, is a mixture of hydrogen (H2) and oxygen (O2) gases produced through the electrolysis of water. This gas is known for its unique properties and applications, including fuel enhancement, welding, and even health benefits. The gas is named after Yull Brown, who popularized its use in various industrial and medical fields.
Brown’s Gas HHO is considered a clean and efficient energy source, as it produces water vapor when combusted, leaving no harmful emissions. It is used in various applications, from improving fuel efficiency in internal combustion engines to providing a high-temperature flame for welding and cutting metals.
-
“The facts behind the media confusion: Yull Brown & the oxy-hydrogen economy” – published in 1978 in Electronics Australia Magazine, pages 14-17, discusses Mr. Yull Brown and his achievements in energy conversion. open here
https://vimeo.com/1007214516?share=copy
When was HHO used for the first time in a vehicle?
The first internal combustion engine was invented by François Isaac de Rivaz. His engine ran on a mixture of hydrogen and oxygen, commonly known as HHO. This groundbreaking invention laid the foundation for modern internal combustion engines.
Is Brown’s Gas HHO more explosive then only Hydrogen?
HHO is a mixture of hydrogen and oxygen in a 2:1 ratio. It is often compared to pure hydrogen in terms of its explosive properties and its unique imploding effect. But is it truly more explosive than just hydrogen?
Watch the videos below for a comparison and demonstration of the explosive properties of Brown’s Gas HHO versus hydrogen.
https://www.youtube.com/watch?v=6gkblppESHAhttps://www.youtube.com/watch?v=qOTgeeTB_kA
Is Brown’s Gas HHO Safe for People?
Brown’s Gas is not dangerous to humans, in fact, it is used in medical circles as a therapeutic gas for inhalation purposes. Brown’s Gas is not dangerous. Obviously, it is flammable in combustion, however, in open air it dissipates quickly. Brown’s Gas is well known to implode rather than explode. The Brown’s Gas flame is a set of implosions as opposed to other fuels that explode.
Benefits of Using Brown’s Gas HHO
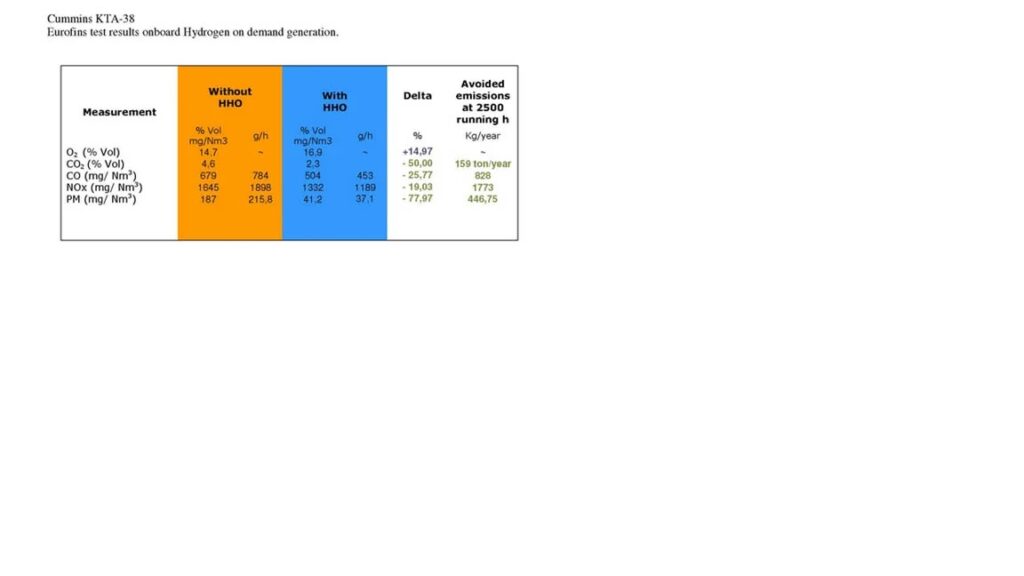
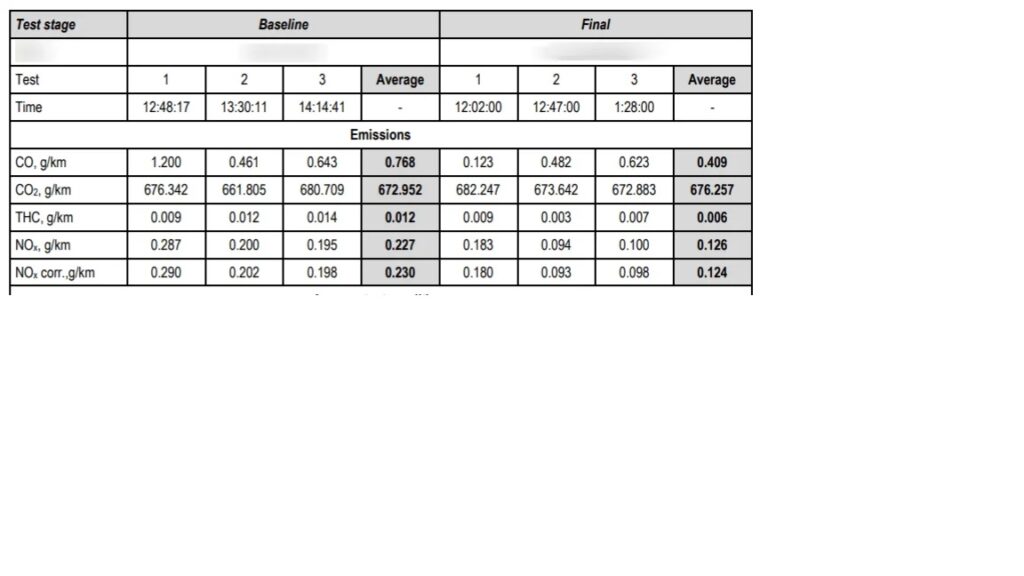
Adding HHO (Brown’s Gas) to an internal combustion engine enhances fuel combustion by increasing flame speed, resulting in more complete fuel burning. This improved combustion efficiency translates to more energy being transferred to the engine and less wasted as heat, positively affecting both power output and fuel economy. Additionally, emissions are reduced, including a significant decrease in hydrocarbons (HC), carbon monoxide (CO), carbon dioxide (CO2), and particulate matter (PM). This cleaner burn also reduces pollutants like nitrogen oxides (NOx), which contribute to smog and acid rain.
In summary, HHO improves engine performance and emissions in several key areas:
- Hydrocarbons (HC): Reduced by 30-40%.
- Oxygen (O2): Increased due to more complete combustion.
- Carbon Monoxide (CO): Reduced by 25-50%.
- Carbon Dioxide (CO2): Reduced by 0-60%. (depends on computer configuration)
- Particulate Matter (PM): Reduced by 70-80%, sometimes over 90%.
- Nitrogen Oxides (NOx): Reduced between 0 – 50%, helping to combat urban pollution.
The fast flame propagation of HHO acts like a powerful spark, igniting all available fuel efficiently.
NOx emissions are closely tied to combustion temperatures, with significant formation occurring above 1527°C (2870°F). As combustion temperatures rise, NOx levels increase substantially. However, adding HHO to the engine can help reduce these emissions by lowering the combustion temperature. The presence of HHO leads to a cooler burn, which reduces the formation of nitrogen oxides. This cooling effect helps mitigate the production of this harmful gas, contributing to a cleaner exhaust profile.
- White Paper: The Characteristics of Brown’s Gas (HHO) in Enhancing Combustion Efficiency. White-Paper-Browns-Gas pdf
- Independant review Dr.-Ellyetts-Report-dated-14Th-August-1987
- Study on Exhaust Gas Characteristics of SI Engine Using Fuel (Gasoline) & HHO Gas
- Effects on Emissions of a Diesel Engine with Premixed HHO
- Efficient Use of Oxy-hydrogen Gas (HHO) in Vehicle Engines (IIETA Report).
*Eurofins is an international group of laboratories headquartered in Luxembourg, providing testing and support services to the pharmaceutical, food, environmental and consumer products industries and to governments.
I’ve heard that such a device uses more energy than it produces.
Our Systems draw about 20 amperes from the vehicle’s electrical system. This requires less than one-horsepower from your engine, a small fraction of the typical power increase provided by the system.
What happens when too much Hydrogen is supplied to an engine?
Using hydrogen or HHO (Brown’s Gas) can improve combustion efficiency in internal combustion engines by helping the fuel burn more completely. However, the benefits have limits. Hydrogen burns roughly 10 times faster than gasoline, so adding too much—whether as pure hydrogen or HHO—can disrupt the engine’s cycle. In gasoline engines, where the spark occurs before the piston reaches the top, excess hydrogen or HHO can ignite too quickly, working against the piston’s upward motion. This premature combustion reduces engine efficiency, causing rough operation and potentially decreasing performance by up to 26%.
HHO, which includes both hydrogen and oxygen, further accelerates the combustion process, making this effect even more pronounced if overused. Proper balance is crucial to avoid counterproductive results, as shown in tests where adding excessive hydrogen or HHO led to a drop in efficiency, despite hydrogen being externally supplied.
What maintanance is required?
To maintain optimal performance, it is recommended to refill the reservoir with demineralized or distilled water every 1000 km, in order that the KOH concentration remains at approximately 5% of the water solution. Additionally, to extend the unit’s lifespan, a periodic system flush is advised every 20.000 km.
Public documents about Brown’s Gas HHO
- Material Today: An Overview of the Analysis of HHO Gas in IC Engines. Click to open pdf.
- Below are videos of vehicles with an HHO addition.
https://youtu.be/uTOddz4x2M8?feature=sharedhttps://www.youtube.com/watch?v=RbDEeOyfmtEhttps://vimeo.com/899653288/1ff8a44d24
Go to shop